Sodium Bicarbonate Decomposition
This was an experiment from our Chemistry class to figure out which of the four equations is the true reaction that we are conducting by burning sodium bicarbonate (baking soda).
Below are the four possible chemical reactions that could be occurring:
- NaHCO3(s) → NaOH(s) + CO2(g)
- 2NaHCO3(s) → Na2O(s) + 2CO2(g) + H2O(g)
- 2NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(g)
- NaHCO3(s) → NaH(s) + CO(g) + O2(g)
We did conduct the fire experiment, but from my predictions, I think the 2nd reaction might be the reaction that may occur…
I learned today that the tongs can be used in two different ways:
- when the tongs are pointed downwards, it is used to pick up the crucible lid (the lid’s handle)
- when the tongs are pointed upwards, the round part of the tongs just clasps around the straight part of the crucible body to pick it up!
I also learned that once the Bunsen burners are turned on, we should immediately ignite them with the striker so that it wouldn’t catch other flammable gases and cause a disaster.

I also learned that the wire-gauze square can actually be used to measure the height between the ring and the fire by rotating it so it is perpendicular to the ground! I also learned that for the heat to equally heat the crucible, we should locate the ring only so that the tip of the fire slightly touches the bottom of the crucible.
Colored fire experiment
This was actually our club experiment, where we experimented with different salt solutions and fire to see the change in color.
Last Friday, some of our club members gathered together in the Chemistry Lab to prepare for the salt solutions for our experiment the following Tuesday. We gathered these materials:
- 50 mL of 1.0M Copper Chloride (CuCl2)
- 50 mL of 1.0M Lithium Chloride (LiCl)
- 50 mL of 1.0M Potassium Chloride (KCl)
- 50 mL of 1.0M Sodium Chloride (NaCl)
- 50 mL of 1.0M Strontium Chloride (SrCl2)
- (Barium Chloride is recommended but it is highly toxic, so we didn’t use it!)
- Distilled water
- 5 weighing boats
- 6, 100 mL beakers
- 1 Graduated Cylinder
- 15 Wooden splints
- 1 Bunsen burner
- 1 Striker


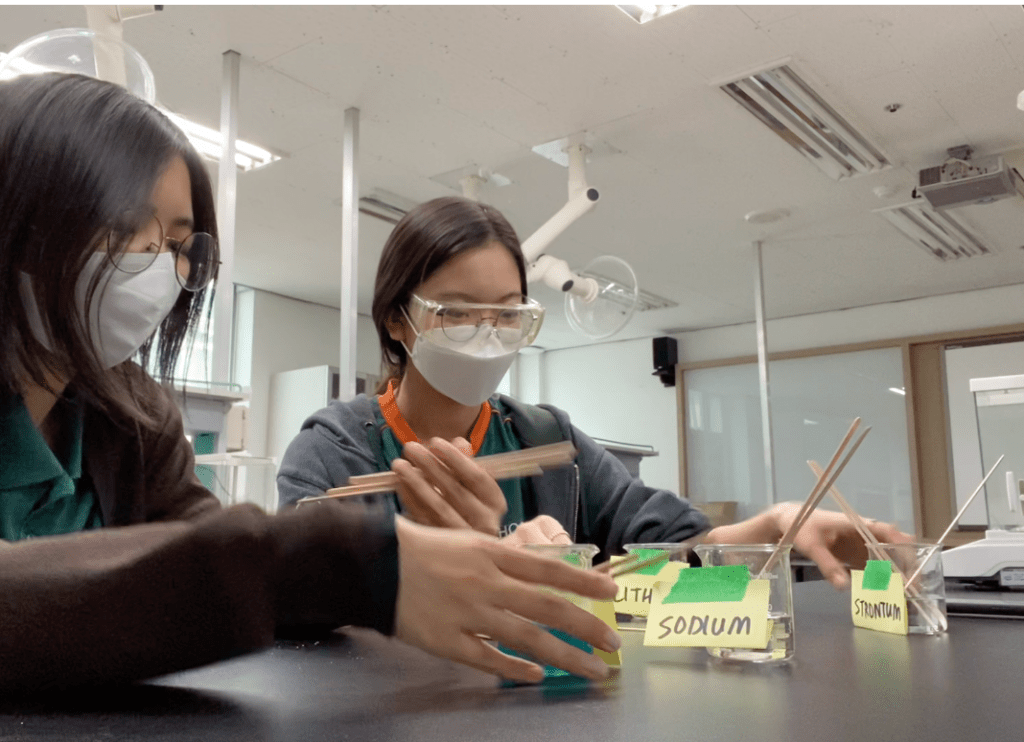




Our salts initially were solid powders, so we measured specific measurements of each salt to a weighing boat and added some distilled water. Then, we dissolved the powder inside the distilled water and labeled the beaker. We added 3 splints into each salt solution and waited over the weekend for the splints to absorb the solutions.
Then, on Tuesday, we started the experiments!
We first practiced using a Bunsen burner, and learned that we should open all windows to ventilate the room for any possible flammations.
These were our findings:
- Strontium – red/orange
- Lithium – pinkish red
- Potassium – Lilac/light purple
- Sodium – bright yellow/orange
- Calcium – bluish-green
We immediately dunked the used splints into the beakers of water to prevent any fires!



















We also learned that fire has different colors depending on its heat – blue fire is hotter than red, which is why the center of the Bunsen burner flame was blue! (it was the hottest)
So, when we placed the splint in the center of the Bunsen burner, the splint burned faster but showed a more vivid color.
We were curious what would happen if we poured the salt solutions straight into the fire? Or what would happen if we spray the solution over the fire? But our chemistry teacher predicted it would actually just extinguish the fire after showing a vivid splash of color.
But this was really interesting to see and also as my first time learning how to use a Bunsen burner, it was a very memorable experience!
– Joanna Kim, May 1rst, 2021, 11:53PM KST