Despite only being able to hold two meetings during March — the other two missing due to the pep rally and spring break, our club was very, very busy.
March 11th
On the first Friday of March, the day before spring break began, we held our second faculty kid session, during which we conducted an elephant toothpaste experiment. Honestly, I could tell the kids were really forward to a very explosive reaction as they were screeching excitedly and fidgeting over their goggles.
However, as we used weaker concentrations of hydrogen peroxide for the kids’ safety, the reaction was not as big and sudden as expected, but the students seemed to really enjoy the activity!


Though we had initially gathered volunteers from our club to guide the event, we ended up having two people in the classroom once the event started as the other volunteers had gone home due to sudden sickness. Thankfully enough, three other members returned to school once they heard missing volunteers, and thanks to them, the event ended successfully.
It’s interesting to see the dynamics of our club members at these service events change over time — many club members struggled initially to effectively communicate with the young children (the youngest one being 2 and the oldest being 10), but as time progressed, I could see more confidence in the eyes of our members, and it also seems as if the children are more familiar with the club members, which actually helps as they’ve become more obedient to safety instructions.
I still remember the first faculty kid session where the children heavily refused and tried to avoid wearing the safety goggles because they carried strange odors (which were from the sterilizer). During this activity, the children still did refuse to wear the goggles, but when we asked them to wear them before and during the chemical reaction of eruption, they actually followed our instructions without much refusal.
March 25th, 2022
LEMON ELECTRICITY EXPERIMENT
It’s officially AP season! During my AP Chemistry class, I recently learned a unit on electrochemistry, and I thought it would be the perfect opportunity to implement what I’ve learned in class and also share with the other members about basic electrochemistry.
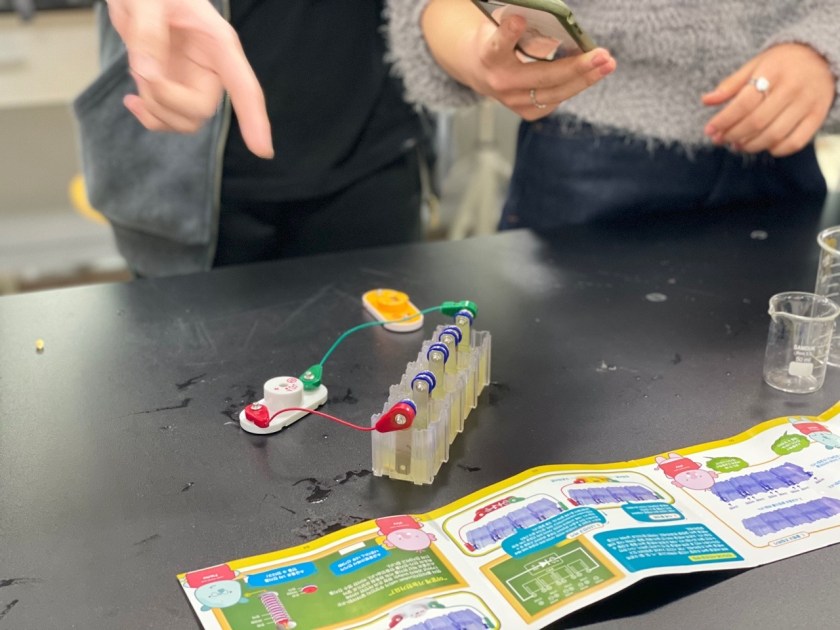
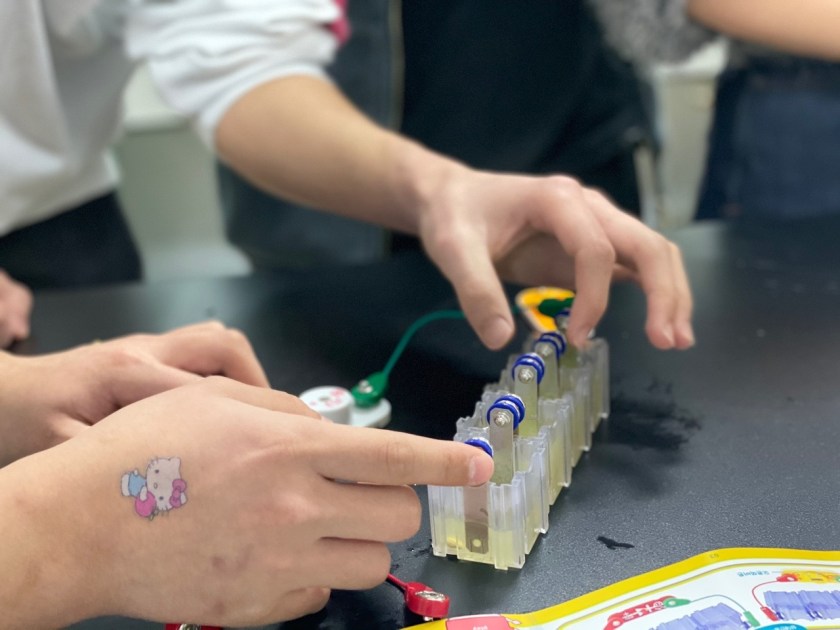
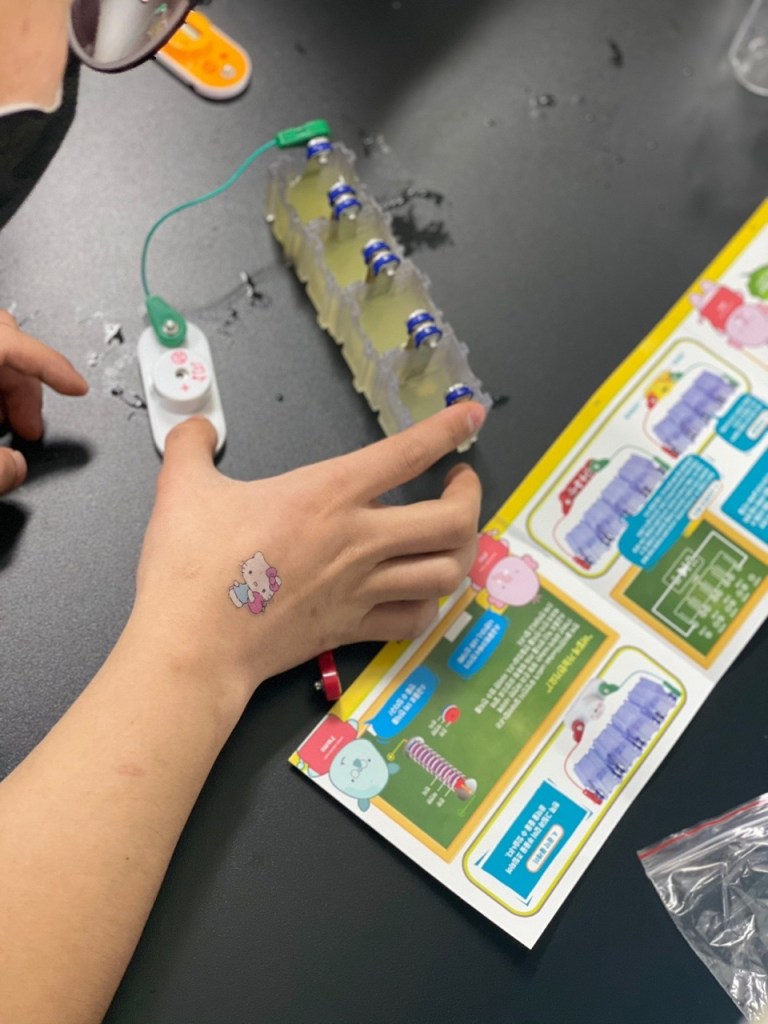
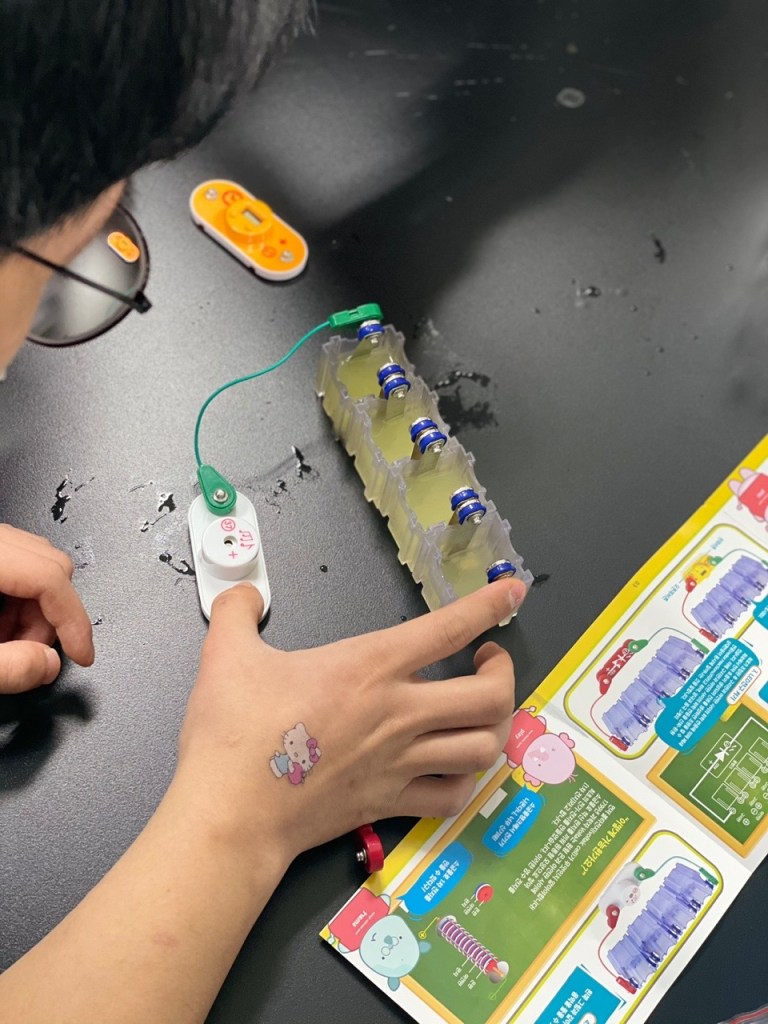
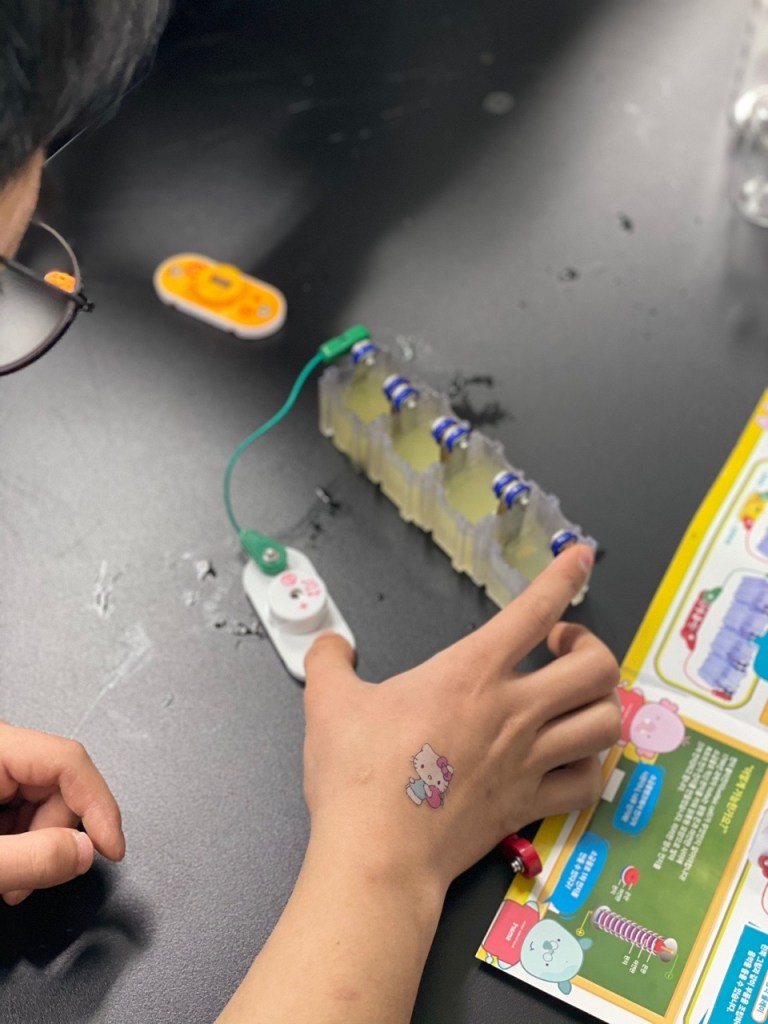
In this experiment, we used copper and iron metals as our electrodes, and lemons to power the clock, lightbulb and music player, which we connected to the electrodes through wire. We used lemons as it was the most common and well known fruit for its acidity, and we knew that the citric acid would be capable of functioning as the electrolyte.
We first had to massage the lemons for the lemon juice to be produced in the lemon. Then, we stuck in the electrode metals on the sides of the lemon, parallel to each other in an alternating pattern. (Iron-copper-iron-copper). Finally, we connected the electrode metals to the lightbulb through wires.
The copper electrode functions as a cathode, undergoing reduction (gaining electrons), whilst the iron functions as an anode, undergoing oxidation (losing electrons). As the citric acid causes the iron to lose electrons, these electrons pass through the wires, converting into electrical energy. Thus, an electrical current is formed and turns on the lightbulb.
Electrochemistry is quite a complex topic, but once you get the idea, you find it interesting (personal opinion).
We tried two ways to test that it was the lemon juice that activated such reactions, not just the presence of a fruit itself. First, we stuck the electrodes in the lemon, and for the second experiment, we squeezed out all the juice from the lemons into small cups, and then placed the electrodes inside. Both reactions worked well, but it seemed as if the lemon juice one may have been more successful in terms of producing more reactions (in the lightbulb, music player, or clock).
One of our members was curious if he could substitute for the wire and placed his fingers on the electrode and the music player, and interestingly, the reaction still occurred.
March 29th
We finally were able to go on the field trip to the museum! Similar to the service event, we had approximately 20 interested members, but ended up only having 9 members who could go, which I’m still very thankful for.
It was nice to see how everyone really enjoyed the field trip, though the heavy traffic and the crammed buses were not expected. Once we arrived at the museum, everyone was panting and sweating from the heavy traffic of people in the bus. Also, I loved the bubbling energy of the members throughout the entire field trip!
One of the members even asked if the field trip was already over. The museum closed at 5:30PM, and we left school at 3:20PM due to a miscommunicated absent member who had expressed interest in going, so despite having a lot of field trip time, the heavy traffic did hinder our way. So, when the club member asked if the field trip was over, everyone was shocked at how quickly the time flew by.
Here are some photos:
– Joanna Kim, March 30th, 2022, 5:14PM KST –