Have you ever felt guilty from stuffing yourself with mouth-watering cookies, or gobbling that one last bite of juicy steak? The impossible urge to resist the temptation of biting down the warm flesh with the aroma of the roasted crust diffusing in your mouth is surely inevitable. But, as of 2016, two billion adults were reported to be overweight, the global prevalence of obesity tripling from 1975 to 2016. Researchers predict that more than one billion adults will be affected by obesity by 2025.
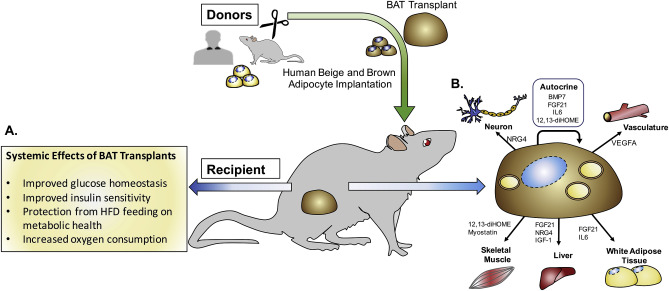
However, there is no need to fear, suggests research by Yu-Hua Tseng, a molecular biologist at Harvard Medical School, where researchers genetically engineered human fat cells using CRISPR-Cas9 in mice to display brown fat-like traits to prevent diet-induced obesity.
CRISPR, short for Clustered Regularly Interspaced Short Palindromic Repeats, is a selective gene-editing tool that is revolutionizing genetic engineering technology. As previous gene-editing technologies were arduous, costly, and less accurate, the development of CRISPR refined the selectiveness of gene selection during trials. As CRISPR utilizes the Cas9 protein, researchers can selectively cut desired segments to disable a particular gene or replace the gap with the desired gene.
Brown fat contains more mitochondria than white fat, which consists of lipids, also known as fatty acids. As brown fat absorbs glucose and white fat and decomposes them using mitochondria to produce energy, they’re able to store more energy in less space than does white fat. Furthermore, a study conducted by Rutgers University discovered that brown fat also filters amino acids from the blood, which are commonly found in eggs, meat, fish, chicken, milk, and muscle-building supplements. Though normal doses of these amino acids are beneficial to the body, excessive amounts of blood are associated with obesity. Thus, the more brown fat one has, the more their bodily systems can filter the amino acids when blood levels exceed, decreasing the vulnerability to obesity and diabetes.
By inserting a molecular switch into the DNA of the white fat cells from subjects’ necks via CRISPR, the researchers were able to activate the UCP1 gene, which initiates energy decomposition in mitochondria in the cell. “Then we transplanted the cells into mice,” says Joslin senior investigator and Harvard Medical School professor Yu-Hua Tseng. This activation of the UCP1 gene significantly increased the amount of brown fat-producing proteins by almost 20 times the average amount. “And we found the mice receiving the transplanted cells had a much-improved metabolism. Even when we gave them a high-calorie, high-fat diet, they gained less weight,” she added.
“I think this could soon become a phenomenal new treatment,” said Corvera, a molecular biologist at the University of Massachusetts Medical School. With this new technology, we can now hope that late-night snacks, Thanksgiving feasts, or that last bite of steak won’t be much of a worry.
– Joanna Kim, May 12th, 2022, 11:50PM KST –