Different gases have different densities. Though we learn in Elementary school that we only exhale carbon dioxide, that claim is partially true. The air we exhale is composed of a variety of different gases, including carbon dioxide, so its density (0.0748 lbm/ft3) is actually lighter than that of carbon dioxide (0.1137 lbm/ft3).
This week, we’re having class with the upper division! The first lesson of week 3 was floating soap bubbles. When I had asked the kids what type of gas we exhale, they all replied Carbon Dioxide (I actually had thought the same too, until I conducted research on it).
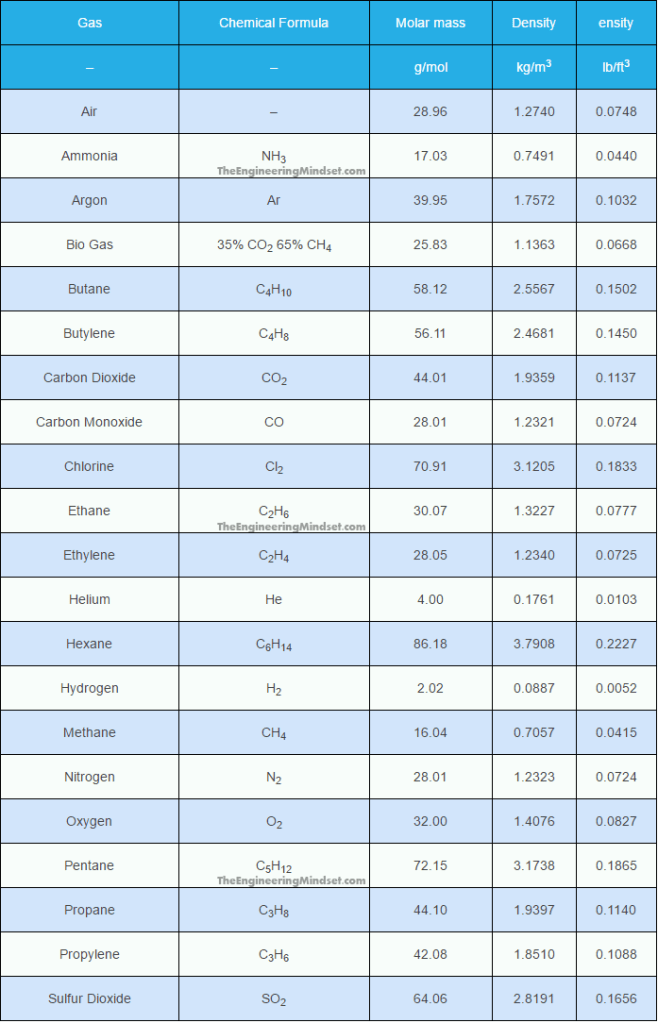
As it can be seen above, air is less denser than carbon dioxide. And, to prove this, we conducted an experiment whereby we would blow bubbles on top of artificially created carbon dioxide gas. The materials of this experiment can be very easily acquired:
- soap bubble solution
- a wand for blowing soap bubbles
- a large transparent container with an open top (an empty 38-liter [10-gallon] aquarium works nicely)
- 125 milliliters (½ cup) of baking soda (sodium bicarbonate)
- 250 milliliters (1 cup) vinegar
In the large tank-like glass container, pour in vinegar. Then, dump in all of the baking soda at once to produce as much carbon dioxide simultaneously as possible. Then, blow bubbles across the top of the container, not directly into the bowl, and watch the bubbles float on top of the gas created from the fizzing solution (mix of vinegar and baking soda).
If you have been reading previous summer camp posts, you probably have seen the acid/base experiments/lessons multiple times. If you haven’t, check these posts out:
ES Summer Camp Day 4 (6/16/22)
Fourth day of summer camp! Today’s lesson was supposed to be focused on a real biodegradation observation, whereby students create their own compost bins, but due to a personal mistake *ahem* of forgetting to remind them to bring their own food waste today, the lesson plan was switched last minute to.. EXPLODING SANDWICH BAGS! Some…
ES Summer Camp Day 8 (6/22/22)
Finally our first experiment for the lower division students! Yesterday, the students decorated their science notebooks. While planning the experiments and lesson plans for the lower division students, who were younger than the upper division students, I often contemplated between whether to use the same lesson plans/experiments as the upper division students or to simplify…
With the purpose of review and improving memory recall, we also had a quiz competition which the kids would win talents if they get placed in the top 3. The quiz basically was “fill in the blanks” of a presentation I made, which we reviewed during class more than twice. The kids were much eager to win the prizes, and I’m glad this motivated them.
This was actually our first failure as an experiment, which I’m also glad to have let the kids experience. Failure in proving the hypothesis correct or achieving the intentional goal of an experiment occurs very frequently amongst researchers and scientists. This was a great learning opportunity for the students and I to learn that sometimes, things won’t work out the way we expect them to.
I think the underlying factor behind our experiment’s failure was the fact that the students blew the bubbles directly into the container, rather than across the top of it. I think the students may have forgotten the instructions once they saw they had to blow across an open-top container, possibly mistaking that they were supposed to blow INTO it. However, it was still very adorable to see the kids have fun with the bubbles after the experiment. The students actually went quite frantic about the bubble solution; they initially were taking turns using the bubble wand, but once one classmate started using their hands to create a circular shape to blow their own bubbles, things got quite out of hand.
On the other hand, some other students were asking me if they could repeat the experiment again. So, we did repeat it, and it didn’t work as well as we expected again. I attribute this erroneous attempt to the size of the container we used, as it wasn’t fully deep enough for the carbon dioxide gas to remain in the walls of the container.
Here are some photos of today’s lesson:
Also, interesting finding! One of the teachers, while searching for another class material, found a female stag beetle in one of the classrooms. She quickly became our class pet, and we named her Binny the Bowser, inspired by the character from the game Super Mario.

– Joanna Kim, August 1rst, 2022, 9:28PM KST –


